Hence in this reaction. Which of the following reactions would result in a.

Which Of The Following Reaction Would Result In A Decrease In Entropy Brainly Com
B COCl2g CO g Cl2g You have 1 mol of gas on the left and 2 mol of gas on the right so entropy is increasing.

. Which of the following reactions shows a decrease in entropy. This is precipitation reaction in which the ions present in solution were in aqueous solution and have more freedom to move here and there so these had more entropy but when these forms the AgClthen these precipitate and the randomness is decreased as these are precipitated in the form of solid. That is when the product is formed from the reactants the randomness of the system decreases as the number of molecules in the product decreases.
CO2 s CO2 9 O C. Decrease in entropy means randomness decreses. Conversely entropy decreases when you have the opposite situations.
A 2COg O2g 2C029 B 2H2O2 2H2Ol O2g с KNO3s Kaq NO3aq D 2N2O3g 2NO2g O2g This problem has been solved. Which of the following reactions would result in decreased entropy. Correct option is A A.
Course Title EXAM 3. A Cs2H2 gCH4 g B H2 OgH2 g12O2 g C 2NI3 sN2 g3I2 g D 2O3 g3O2 g E None of the above Easy Open in App Solution Verified by Toppr Correct option is A Option Ais the correct answer. N2204 9 2NO39 maxcossagolia2004 is waiting for your help.
See the answerSee the answerSee the answerdone loading Show transcribed image text Expert Answer Who are the experts. Only in the case of the first reaction the reactant has three molecules whereas the product has only one molecule. KNO3 s K aq NO3- aq People also asked Which of the following.
Which of the following reactions would result in a decrease of the entropy from EXAM 3 at University of Texas. Hence entropy also decreases. School University of Texas.
A chemical reaction that has a positive delta G. Which reactions would result in increased entropy. Entr View the full answer.
QuestionWhich of the following reactions will result to a decrease in entropy. H20 g H20 1 O B. This is the correct answer.
While other options reactants and products are in. Match the following List 1 Entropy of vaporisation K for spontaneous process Crystalline solid state Delta U in adiabatic expansion of ideal gas List 2 decreases is always positive lowest entropy dfracDelta H_text vap T_text b. Multiple choice 2 See answers Advertisement.
Advertisement Advertisement bradbennett400. Which of the following reactions will result in a decrease in entropy of the system. The reaction that would result in decreased entropy is N2 3H2 2NH3.
A 2NO gO2g 2NO2g You have 3 mol of gas on the left and 2 mol on the right so entropy is decreasing. Since the state of matter is going from a gas to a solid it would be going from a state of high entropy greater disorder to a state of lower entropy less disorder. O NH Cls - NH3g HCls O 2 Hals O2g 2 H00 O 2 N Hale -2 NH3g Hale ONO4s 2 NO6 O NaCls Na aq Claq.
Which of the following reactions would result in decreased entropy. Because recatants are in solid form having less enetropy and products are liquid form having more entropy. The types of reaction that would decrease the entropy within a cell is dehydration reactions.
D CaOs CO2g CaCO3s Entropy means randomness. Increase in entropy means the molecules of compound move more freely. 203 9 302 9 O D.
Add your answer and earn points.

Which Of The Following Reactions Would Result In Decreased Entropy Multiple Choice Brainly Com
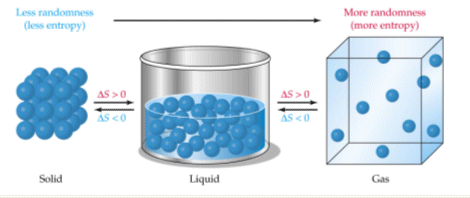
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic

Chemical Thermodynamics Ppt Download

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
0 Comments